Biocidal Products
Biocidal Products
- Products subject to any of the following that aim to remove hazardous organisms, etc.
- A product composed of one or more active substances, or a product with a mixture of an active substance and non-active substance
- A product generating an active substance from a chemical substance or a mixture of a chemical substance and natural substance or a microbe
information Active substances or biocidal products manufactured or imported in Korea must receive approval before being sold/distributed in Korea
- Manufacture, import, sales, and distribution of biocidal product that has not received the approval of the Ministry of Environment are prohibited
Approval Procedure of Biocidal Products
- Application for approval, assessment, approval, etc., are based on the Chemical Product Management System (CHEMP)
- Applications are divided into single product and biocidal product family
- It takes 12 months from the application for approval to the notification of approval (biocidal product)
- Preparation of “Biocidal Product Dossier” (BPD)
- Hazard confirmation data (test data, non-test data, general data)
- Application for approval of biocidal product
- Response to the National Institute of Environmental Research through modification and supplementation
Flowchart of Biocidal Product Approval
Approval Procedure of Biocidal Products
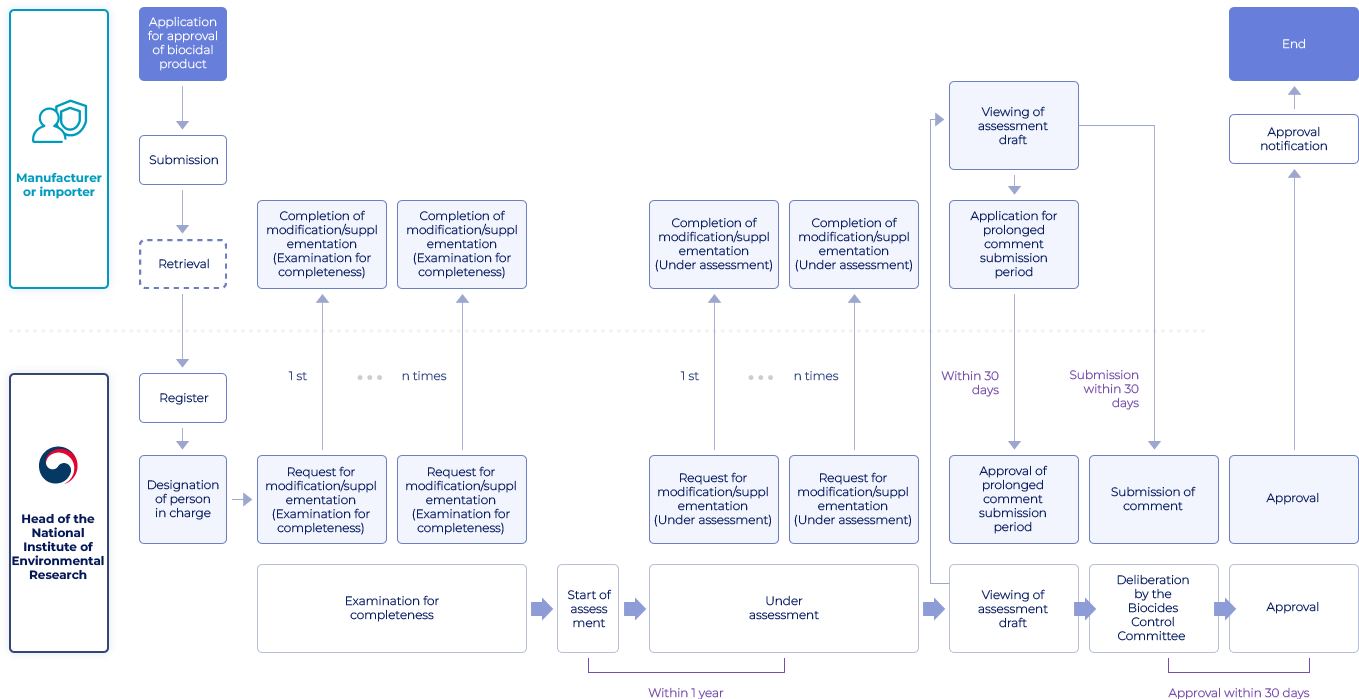
- Manufacturer or importer
- Application for approval of biocidal product → Submission
- Submission → Retrieval
- Retrieval → Register
- Completion of modification/suppl ementation (Examination for completeness)
- Viewing of assessment draft → Application for prolonged comment submission period, Viewing of assessment draft → Submission of comment(Submission within 30 days)
- Application for prolonged comment submission period → Approval of prolonged comment submission period(Within 30 days)
- Approval notification → End
- End
- Head of the National Institute of Environmental Research
- Register → Designation of person in charge
- Designation of person in charge → Request for modification/suppl ementation (Examination for completeness)
- Request for modification/suppl ementation (Examination for completeness) → Completion of modification/suppl ementation (Examination for completeness)(1st, ...n times)
- Approval of prolonged comment submission period
- Submission of comment
- Approval → Approval notification
- Examination for completeness → Start of assess ment
- Start of assess ment(Within 1 year) → Under assessment
- Under assessment(Within 1 year) → Viewing of assessment draft
- Viewing of assessment draft → Viewing of assessment draft, Viewing of assessment draft → Deliberation by the Biocides Control Committee
- Deliberation by the Biocides Control Committee → Approval(Approval within 30 days)
- Approval(Approval within 30 days)
Biocidal Product Approval Tasks
- Preparation of biocidal product approval data
- Preparation of biocidal product similarity data
- Component analysis, accelerated storage, effect and efficacy test application according to biocidal product
- Application for tests on physical and chemical properties, hazard to human body, and hazard to environment according to biocidal product
- Hazard assessment on biocidal products
- Examination of biocidal product labeling matters
- Components and mixture ratio of all active substances used for the biocidal product
- Name or company name, address, and contact information of the manufacturer or importer who received the approval for the biocidal product
- Risks according to the use of the biocidal product and first aid method
- Expiry date and disposal method of the biocidal product
- In the case where nanomaterial is intentionally contained in the biocidal product, the name, purpose, and use of the substance