Registration/Notification
Overview of Registration/Notification Under K-REACH
Korean Manufacture/Import
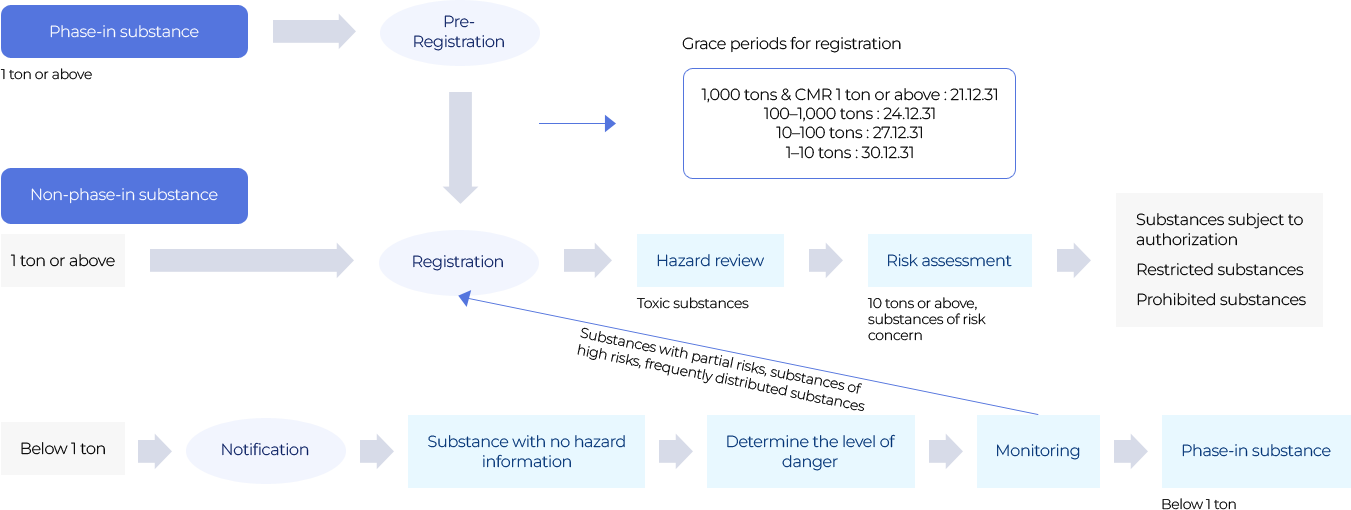
- Phase-in substance(1 ton or above) >(on the) Pre-Registration >(on the) Registration
- Pre-Registration >(on the) Grace periods for Registration
- Grace periods for Registration
- 1,000 tons & CMR 1 ton or above : 21.1231
- 100-1,000 tons : 24.1231
- 10-100 tons : 27.1231
- 1-10 tons : 30.1231
- Non-phase-in substance(1 ton or above) >(on the) Registration >(on the) Hazard review(Toxic substances(1,000 types)) >(on the) Risk assessment(10 tons or above, substances of) >(on the) Substances subject to authorization, Restricted substances(13 types), Prohibited substances(60 types)
- Below 1 ton >(on the) Notification >(on the) Substance with no hazard information >(on the) Determine the level of denger >(on the) Monitoring (Substances with partial risks, substances of high risks, frequently distributed substances ask Registration) >(on the) Phase-in substance(Below 1 ton)
Registration/Notification Procedure
Preparation and submission of documents required for registration
-
Request Consultation for
Registration of chemical
substance -
Receive Basic Information
and provide a quotation -
Preparation and
submission of dossiers -
Report application
results and provide
post-management
Services Provided for Registration/Notification
-
Management of Phase-in
Substance Consortia -
Consulting on non-phase-in
substances -
Expert
Advisory Service